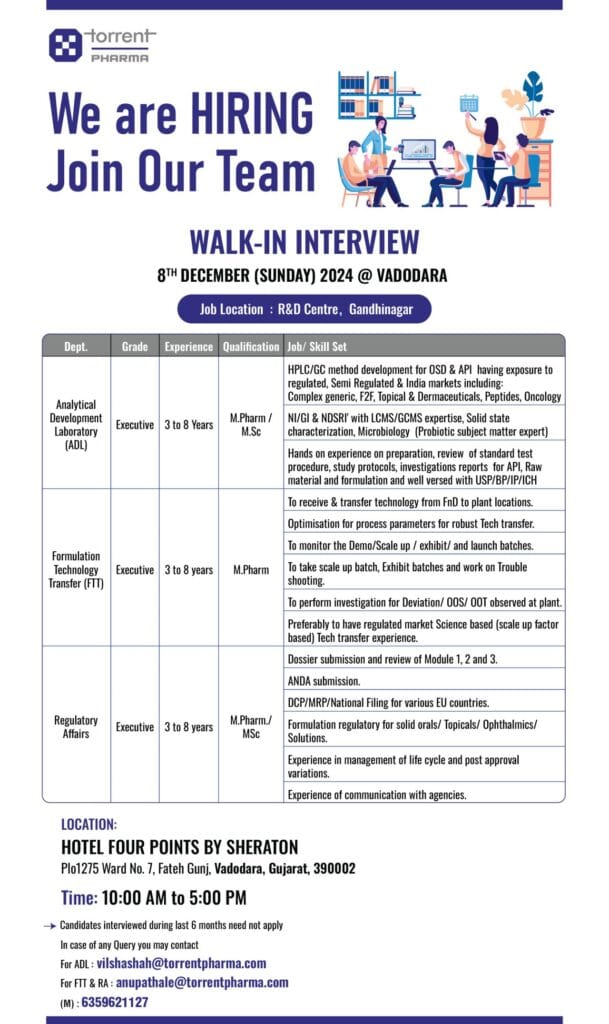
Torrent Pharma R&D Centre Gandhinagar . Pharmaceutical Walk In Interviews For Experienced ( Pharma Jobs ).
Pharmaceutical Walk-In for Torrent Pharma R&D Centre Gandhinagar .
Organization Profile
Torrent Pharma, the flagship Company of Torrent Group is one of the leading pharma companies of the Country. The Company was a pioneer in initiating the concept of niche marketing in India and today is ranked amongst the leaders in therapeutic segment of cardiovascular (CV), central nervous system (CNS), gastro-intestinal (GI) and women healthcare (WHC). The Company also has significant presence in diabetology, pain management, gynaecology, oncology and anti-infective segments. Torrent Pharma’s competitive advantage stems from the world-class manufacturing facilities, advanced R&D capabilities, extensive domestic network and a widespread global presence in over 50+ countries.
Pharmaceutical Walk-In Details
- Job Location – Torrent Pharma R&D Centre , Gandhinagar .
- Qualification – M. Pharma / M. Sc .
- Walk-in Date & Time – 08 Dec 2024 ( Sunday ) Time – 10:00 am to 05:00 pm .
- Interviews Venue – Hotel Four Points By Sheraton , 1275 Ward, No. 7, Fateh Gunj, Vadodara, Gujarat 390002 .
Torrent Pharma R&D Centre Gandhinagar .
Hiring Note
Candidates Interviewed During Last 6 Months Need Not Apply .
In Case Of Any Query You May Contact
For ADL : vilshashah@torrentpharma.com
For FTT & RA : anupathale@torrentpharma.com
Mob : 6359621127
Vacancy Details
- Department – ADL / FTT / Regulatory Affairs .
- EXP – 03 to 08 years .
- Positions – Executive .
Job Description
- ADL– HPLC/GC method development for OSD & API having exposure to regulated, Semi Regulated & India markets including: Complex generic, F2F, Topical & Dermaceuticals, Peptides, Oncology . NI/GI & NDSRI’ with LCMS/GCMS expertise, Solid state characterization, Microbiology (Probiotic subject matter expert) . Hands on experience on preparation, review of standard test procedure, study protocols, investigations reports for API, Raw material and formulation and well versed with USP/BP/IP/ICH .
- FTT – To receive & transfer technology from FnD to plant locations. Optimisation for process parameters for robust Tech transfer. To monitor the Demo/Scale up / exhibit/ and launch batches. To take scale up batch, Exhibit batches and work on Trouble shooting. To perform investigation for Deviation/ OOS/ OOT observed at plant. Preferably to have regulated market Science based (scale up factor based) Tech transfer experience.
- R&D – Dossier submission and review of Module 1, 2 and 3. ANDA submission. DCP/MRP/National Filing for various EU countries. Formulation regulatory for solid orals/ Topicals/ Ophthalmics/ Solutions. Experience in management of life cycle and post approval variations. Experience of communication with agencies.